Research Summary
Functional responsibilities of cathepsin G (CatG) in immunity, including bivalent regulation of major histocompatibility complex class I (MHC) molecules, which underscore a novel role of CatG within the immune system.
Special Interests: Immune evasion of tumor cells and pathogens, modulating of the proteolytic activity of CatG, functional properties of CatG in T cells, immunosenescence
Timo Burster, Associate Professor Email: timo.burster@nu.edu.kz

Fig. 1: Three-dimensional structure of human cathepsin G (CatG).
The catalytic triad is shown: H57, D102, and S195 and the N-linked glycosylation site is found at N65. The polymorphism within the coding region is labeled as N119S. PDB: 1CGH P. Based on Hof, I et al., EMBO J, 15 (1996) 5481-5491.
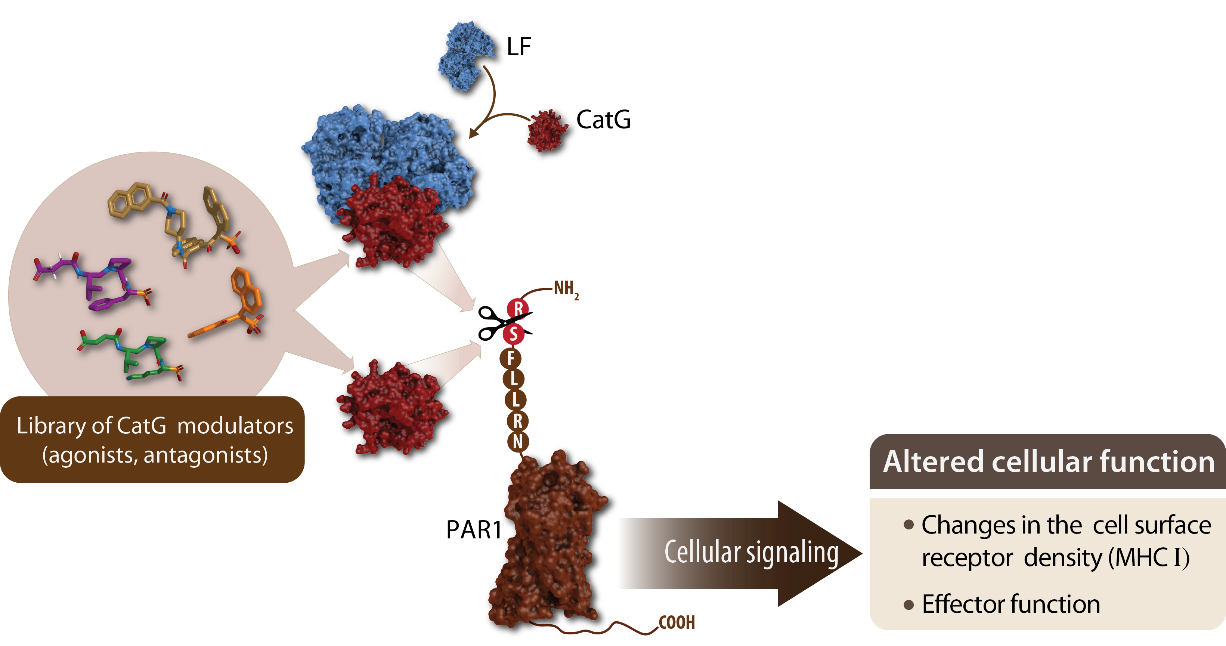
Fig. 2: A model of how exogenous CatG modulates cell surface expression of MHC I.
CatG proteolytically cleaves the N-terminal end of the extracellular domain of protease-activated receptor 1 (PAR1). As a result, the tethered activation ligand flips to the extracellular loop 2 and recruits intracellular G protein, which is capable of downstream signal transduction.
However, cleavage of the extracellular part of PAR1 by CatG can also lead to receptor inactivation, so-called dis-arming; thereby the tethered ligand as well as three extracellular loops of PAR1 are digested and blocks G protein-mediated signaling. This can be further enhanced by the interaction of lactoferrin (LF) with CatG. LF increases the proteolytic activity of CatG and augments a CatG-mediated upregulation of MHC I molecules on the cell surface. The MHC I recycling pathway, for instance, is important for loading a new set of antigenic peptides (viral derived or tumor-associated) to MHC I molecules in order to be displayed on the cell surface for CD8+ T cell inspection. Distinct viruses or tumor cells prevent the synthesis of nascent MHC I molecules or interfere with the MHC I recycling pathway. The advantage of CatG-mediated upregulation of cell surface MHC I molecules is the efficacy to reuse MHC I within the MHC I recycling pathway, where MHC I molecules are pushed out to the cell surface instead of being degraded in the lysosome, as an additional model regarding how CatG circumvents immune evasion of viruses or tumor cells.
Publications
Recent publications
- Roman Schroeder, Renata Grzywa, Christian Rainer Wirtz, Marcin Sienczyk, and Timo Burster. Application of a novel FAM-conjugated activity-based probe to determine cathepsin G activity intracellularly. Analytical Biochemistry: Methods in the Biological Sciences. 2020. 588, 113488. DOI: 10.1016/j.ab.2019.113488.
- Bożena Obmińska-Mrukowicz, Marianna Szczypka, Magdalena Lis, Aleksandra Pawlak, Agnieszka Suszko-Pawłowska, Angelika Sysak, Aleksandra Zambrowicz, Timo Burster, Maja Kocięba, Jolanta Artym, Ewa Zaczyńska, Iwona Kochanowska, Michał Zimecki. Effects of Yolkin on the Immune Response of Mice and Its Plausible Mechanism of Action. Immunology Letters. 2020. DOI: 10.1016/j.imlet.2020.01.003.
- Adriane Penczek and Timo Burster. Cell surface cathepsin G can be used as an additional marker to distinguish T cell subsets. Biomedical Reports. 2019. Apr; 10 (4):245-249. DOI: 10.3892/br.2019.1198.
- Lubos Marta, Dębowski Dawid, Barcinska Ewelina, Meid Annika, Inkielewicz-Stepniak Iwona, Burster Timo, and Rolka Krzysztof. Inhibition of human constitutive 20S proteasome and 20S immunoproteasome with novel N-terminally modified peptide aldehydes and their antitumor activity. Peptide Science. 2019. DOI: 10.1002/pep2.24100.
Reviews
- Joachim Bischof, Fabian Gärtner, Katja Zeiser, Rebecca Kunz, Corinna Schreiner, Elena Hoffer, Timo Burster, Uwe Knippschild, and Michal Zimecki. Immune cells and immunosenescence. Folia Biologica. 2019. 65(2):53-63.
- Pengfei Xu, Chiara Ianes, Fabian Gärtner, Congxing Liu, Johannes Lemke, Timo Burster, Vasiliy Bakulev, Najma Rachidi, Uwe Knippschild, Joachim Bischof. Regulation and functions of the stress-induced CK1 delta: A promising therapeutic target in neurodegenerative diseases and Cancer. Gene. Review. 2019. Oct 5;715:144005. doi: 10.1016/j.gene.2019.14400
All publications
Pubmed
Google Scholar
Contact Information
E-mail: timo.burster@nu.edu.kz
Three-dimensional structure of human cathepsin G.
The catalytic triad is shown: H57, D102, and S195 and the N-linked glycosylation site is found at N65. The polymorphism within the coding region is labeled as N119S. PDB: 1CGH P. Based on Hof, I et al., EMBO J, 15 (1996) 5481-5491.




