
Research Interest
Currently, Dr. Fan-Hagenstein is interested in the research mainly on the following two aspects.
1. Study of the intermolecular interactions in the solution phase using the combination of quantum mechanical calculations and vibrational spectroscopy.
2. Synthesis, characterization, and application of carbon-based nanodots (C-dots) and their aggregations.
Dr. Haiyan Fan-Hagenstein received her Ph.D. in Physical Chemistry from Marquette University. Her doctoral research in the group of Dr. Scott Reid focused on the study of the fine and hyperfine rovibronic structure of some interstellar or combustion reaction related to small radicals and carbenes using techniques including jet cooled molecular beam, laser-induced fluorescence, and quantum beat measurement. She then spent a few years as a postdoctoral appointee at Argonne national laboratory in Dr. Stephen Pratt’s group studying the vacuum ultraviolet and multiphoton photoionization dynamics of atoms or radical products of photodissociation, photoionization, and photodissociation from excited states, and autoionization and predissociation phenomena using ion imaging techniques. The postdoctoral experience at Concordia College in the group of Dr. Darin Ulness led her to the study of hydrogen or halogen bonding in the solution phase using Raman, FTIR, and NMR spectroscopy.
Her research focuses mainly on two aspects:
- Study of the intermolecular interactions in the solution phase using the combination of quantum mechanical calculations and vibrational spectroscopy.
In this part of the study, they focus on various intermolecular interactions including hydrogen bonding, halogen bonding, and general van der Waals interaction based on the vibrational frequency shift reflected in the FT-IR and Raman spectroscopy. In the following, they will present some examples to show the progress they have achieved with this respect.
a) Among 27 vibrational normal modes, they classified 7 Ring related Normal modes (RNMs) that take more than 40% contribution from the ring bond distances and angles. These 7 RNMs indicate strong sensitivity toward the change in the electronic structure induced by the intermolecular interactions and/or substitution on the pyridine ring. Such classification laid a strong foundation for the study of pyridine involved intermolecular interaction, the characterization of the pyridine derivatives in the organic synthesis, and the interpretation of anharmonicity reflected in the pyridine related vibrational spectroscopy (Reference: E. Benassi, H. Fan. Quantitative characterisation of the ring normal modes. Pyridine as a study case. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 246 (2021) 119026)
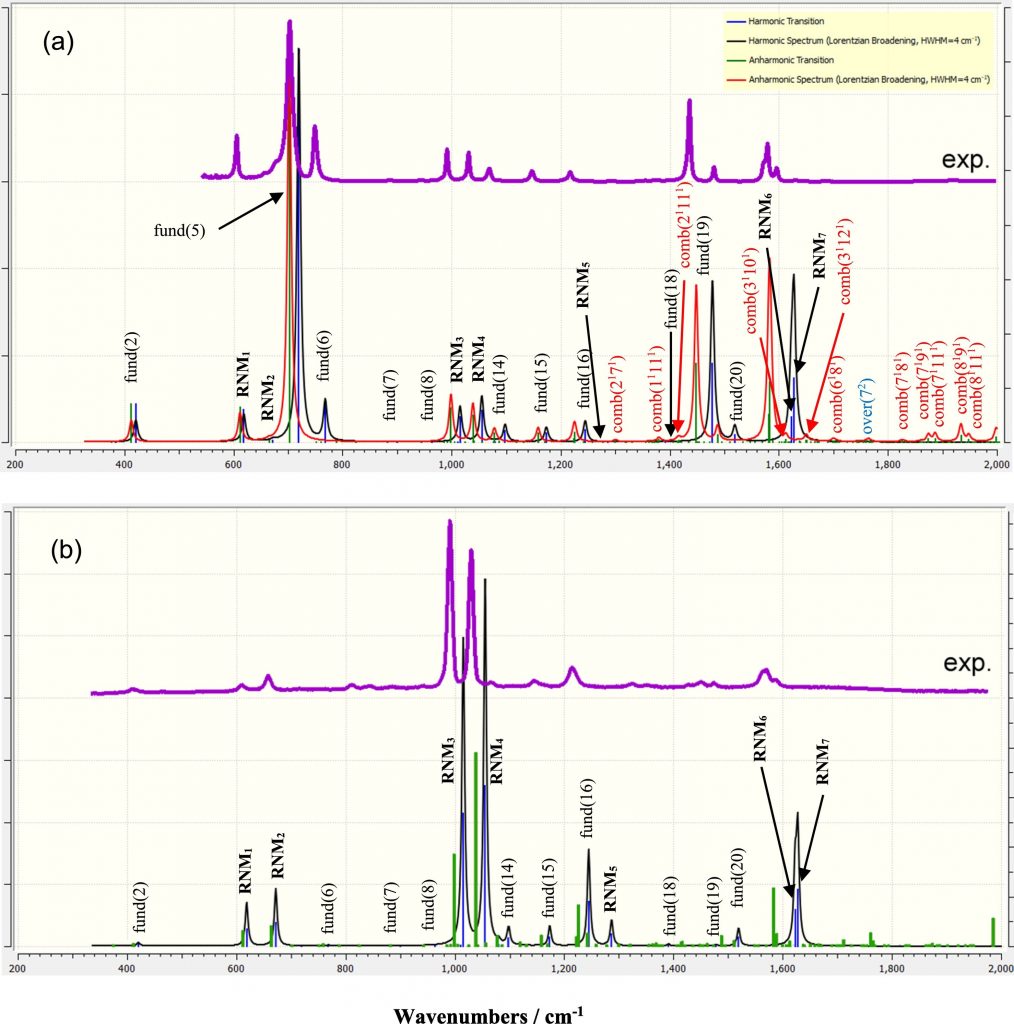
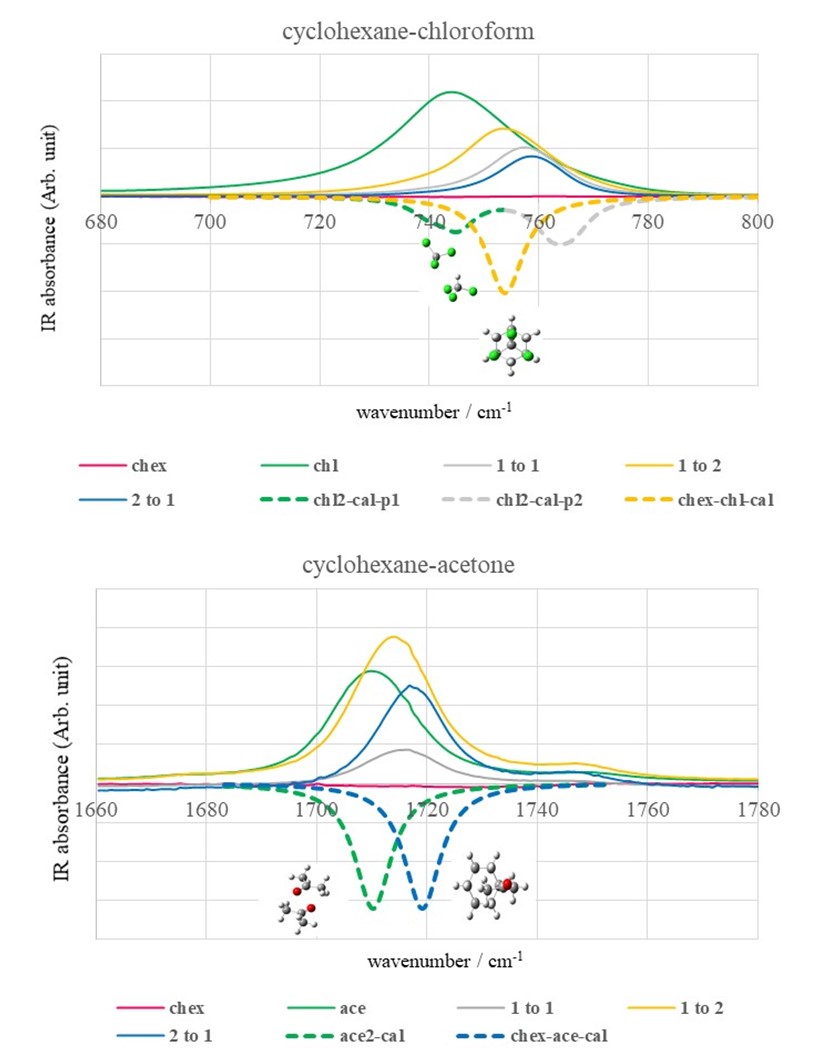
b) The shift in the vibrational frequency was identified even in the binary organic systems made of cyclohexane and another polar organic solvent, wherein the intermolecular interaction is dipole-induced dipole natured van der Waals interaction.
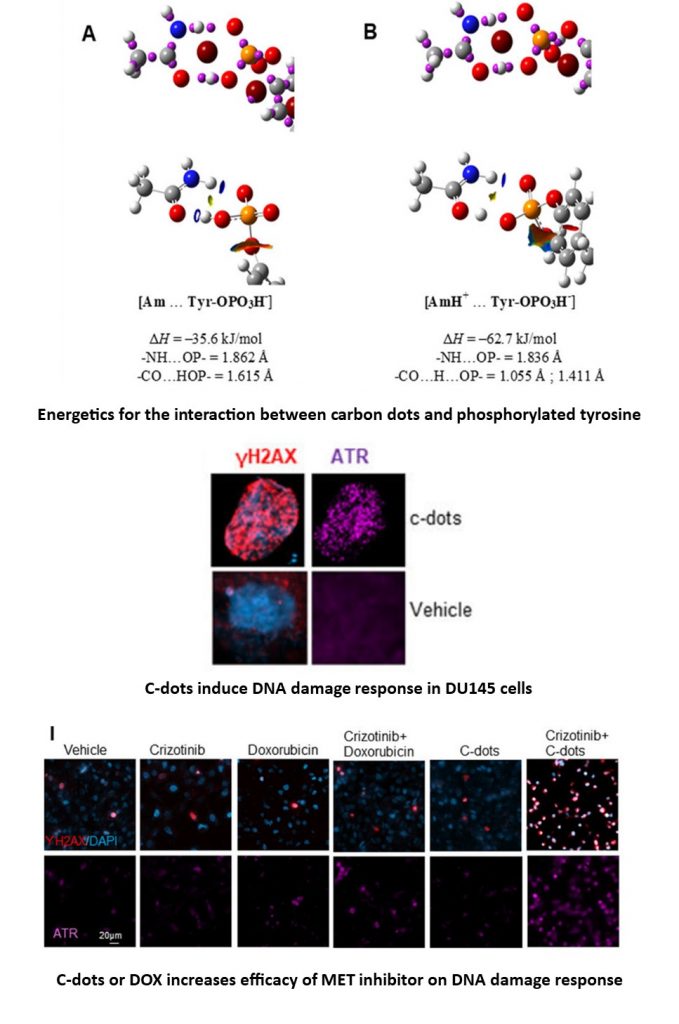
2. Synthesis, characterization, and application of carbon-based nanodots (C-dots) and their aggregations.
In this project, they use vegetables such as beet, cauliflower, and soybean as the starting materials, combining the adoption of N and S to develop some carbon-based nanomaterials through a simple hydrothermal treatment. Some of these materials indicated anticancer and antibacterial properties. More importantly, they took advantage of the quantum mechanical calculation by using a scaffold containing the functional groups like amide, carboxylic, or thiol groups to represent the carbon nanoparticles to study their potential interaction with DNA, cell membrane, and enzymes. (Reference: Y. Xie, H. Fan, W. Lu, Q. Yang, A. Nurkesh, T. Yeleussizov, A. Maipas, J. Lu, L. Manarbek, Z. Chen, E. Benassi. Nuclear MET requires ARF and is inhibited by carbon nanodots through binding to phospho-tyrosine in prostate cancer. Oncogene 38 (2019) 2967)
Fall 2023
- CHEM 331 – Physical Chemistry I
- CHEM 33L – Physical Chemistry I Lab
- CHEM 510 – Principles of Physical Chemistry
- CHEM 730 – Chemical Thermodynamics and Kinetics
- CHEM 591 – Scientific Methods in Chemistry (team-taught course)
CHEM 700 – Hot Topics in Chemistry (team-taught course)
Spring 2023
- CHEM 332 – Physical Chemistry II
- CHEM 332L – Physical Chemistry II Lab
Fall 2022
- CHEM 331 – Physical Chemistry I
- CHEM 33L – Physical Chemistry I Lab
- CHEM 510 – Principles of Physical Chemistry
Spring 2022
- CHEM 331L – Physical Chemistry I Lab
- CHEM 332 – Physical Chemistry II
- CHEM 332L – Physical Chemistry II Lab
Fall 2021
- CHEM 331 – Physical Chemistry I
- CHEM 510 – Principles of Physical Chemistry
Summer 2021
- CHEM 101L – General Chemistry I Lab




